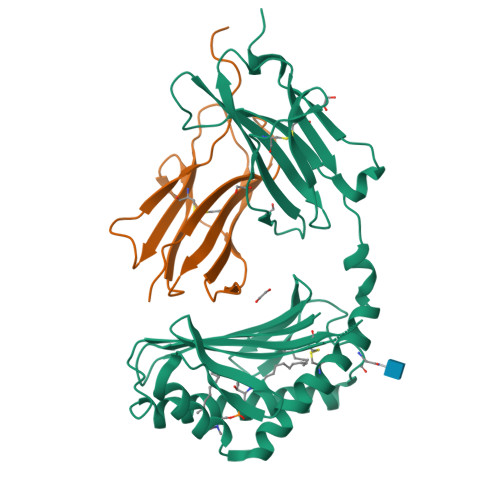
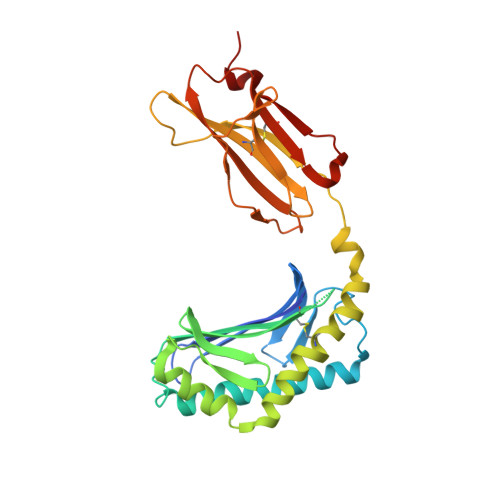
CD1a selectively captures endogenous cellular lipids that broadly block T cell response.
Cotton, R.N., Wegrecki, M., Cheng, T.Y., Chen, Y.L., Veerapen, N., Le Nours, J., Orgill, D.P., Pomahac, B., Talbot, S.G., Willis, R., Altman, J.D., de Jong, A., Van Rhijn, I., Clark, R.A., Besra, G.S., Ogg, G., Rossjohn, J., Moody, D.B.(2021) J Exp Medicine 218
- PubMed: 33961028
- DOI: https://doi.org/10.1084/jem.20202699
- Primary Citation of Related Structures:
7KOZ, 7KP0, 7KP1 - PubMed Abstract:
We optimized lipidomics methods to broadly detect endogenous lipids bound to cellular CD1a proteins. Whereas membrane phospholipids dominate in cells, CD1a preferentially captured sphingolipids, especially a C42, doubly unsaturated sphingomyelin (42:2 SM). The natural 42:2 SM but not the more common 34:1 SM blocked CD1a tetramer binding to T cells in all human subjects tested. Thus, cellular CD1a selectively captures a particular endogenous lipid that broadly blocks its binding to TCRs. Crystal structures show that the short cellular SMs stabilized a triad of surface residues to remain flush with CD1a, but the longer lipids forced the phosphocholine group to ride above the display platform to hinder TCR approach. Whereas nearly all models emphasize antigen-mediated T cell activation, we propose that the CD1a system has intrinsic autoreactivity and is negatively regulated by natural endogenous inhibitors selectively bound in its cleft. Further, the detailed chemical structures of natural blockers could guide future design of therapeutic blockers of CD1a response.
Organizational Affiliation:
Graduate Program in Immunology, Harvard Medical School, Boston, MA.